m-Anisidine
What is M-Anisidine, cas no:536-90-3,a producer telling you the result.
CAS NO.536-90-3
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
To begin with, let us tell you what is the basic information of M-Anisidine ?
|
Molecular Formula |
C7H9NO |
Molecular Weight |
123.152 |
|
Density |
1.1±0.1 g/cm3 |
Boiling Point |
|
|
Flash Point |
109.6±13.1 °C |
Melting Point |
−1-1 °C(lit.) |
Like many stuff, it has many synonyms as follows
|
3-Methoxyaniline |
|
3-Aminoanisole |
|
M-METHOXYANILINE |
|
m-Aminoanisole |
|
m-Anisidine |
|
3-anisidine |
First, the chemical is very special, some technical indexes as below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Physical property data
1. Appearance: Light yellow oily liquid.
2. Density (g/cm3, 16 ℃): 1.094
3. Relative vapor density (g/cm3, air=1): undetermined
4. Melting point (º C):- 1~1
5. Boiling point (º C, atmospheric pressure): 251
6. Boiling point (º C, 8kPa): undetermined
7. Refractive index (n20D): 1.581
8. Flash point (º C):> one hundred and ten
9. Specific rotation (º): not determined
10. Spontaneous combustion point or ignition temperature (º C): not determined
11. Vapor pressure (mm Hg, 20 º C): not determined
12. Saturated vapor pressure (kPa, 55.1 º C): undetermined
13. Combustion heat (KJ/mol): undetermined
14. Critical temperature (º C): not determined
15. Critical pressure (KPa): undetermined
16. Logarithmic value of oil-water (octanol/water) partition coefficient: undetermined
17. Explosion upper limit (%, V/V): undetermined
18. Lower explosive limit (%, V/V): not determined
19. Solubility: soluble in ethanol, ether, benzene, and dilute acid, slightly soluble in water
toxicology data
1. Acute toxicity: Quail oral LD50: 562mg/kg, no detailed explanation except for lethal dose;
Oral LD50 for wild birds: 562mg/kg, with no detailed explanation except for lethal dose;
1. Mutation data: Cytogenetic analysisTEST system: Rodent hamster ovary: 160mg/L;
Sisters chromatid exchangeTEST system: rodent hamster ovary: 50mg/L;
Ecological data
Molecular structure data
1. Molar refractive index: 37.16
2. Molar volume (cm3/mol): 115.7
3. Waiting ratio (90.2K): 289.7
4. Surface tension (dyne/cm): 39.3
5. Polarization rate (10-24cm3): 14.73
Calculate chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: None
6. Topological molecular polarity surface area 35.2
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 85
10. Number of isotopic atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Nature and stability
1. Use and store according to specifications, without decomposition, and avoid contact with oxides.
2. Irritating to the respiratory system and harmful by inhalation.
3. Exists in spice tobacco leaves and smoke.
Storage
1. Store in a cool, dry, and well ventilated warehouse. Stay away from sources of fire and heat. Protect from direct sunlight. Sealed packaging. It should be stored separately from acidic and edible chemicals, and avoid mixing storage. The storage area should be equipped with suitable materials to contain leaked materials.
Second, the Synthetic Route we will recommend is the most important for your reference?
First, synthesis line ofM-Anisidine CAS NO.536-90-3 as follows
Synthetic method
1. Obtained by methylation of meta nitrophenol on the hydroxyl group and reduction.
2. Tobacco: OR,18
Third, what is the usage of M-Anisidine CAS NO.536-90-3 ? pleas see below
If you need the products .Please send your inquiry to us through e-mail:sales@yuansu-reagent.com
Usage: 1. Meta aminobenzyl ether is a drug, dye intermediate, polymer material, aluminum alloy corrosion inhibitor, heavy metal ion chelation analysis reagent, and reducing agent for photosensitive materials.
2. Biochemical research, organic synthesis.
Main usages:
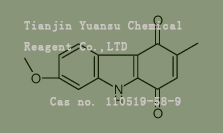
Used for the synthesis of 7-methoxy-3-methyl-9H-carbazole-1,4-dione Cas no. 110519-58-9
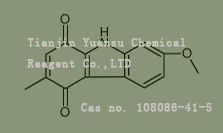
Used for the synthesis of N-(3-methoxyphenyl)-2-(4-oxoquinazolin-3-yl)acetamide Cas no. 108086-41-5
Used for the synthesis of 2-(3-methoxyphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane Cas no. 325142-84-5
Besides Safety Information ofM-Anisidine CAS NO.536-90-3 is also important when handling it
|
Hazard Codes |
Xi |
|
WGK Germany |
3 |
|
H.S.Code: |
2922 2990.90 |
|
TSCA |
Yes |
|
HazardClass |
IRRITANT |
What is the appearance ofM-Anisidine CAS NO.536-90-3? Please see the picture ofM-Anisidine CAS NO.536-90-3, below
If you need the products .Please send your inquiry to us through e-mail: sales@yuansu-reagent.com
Specification ofM-Anisidine CAS NO.536-90-3, is below
Apperance: Light yellow oily liquid
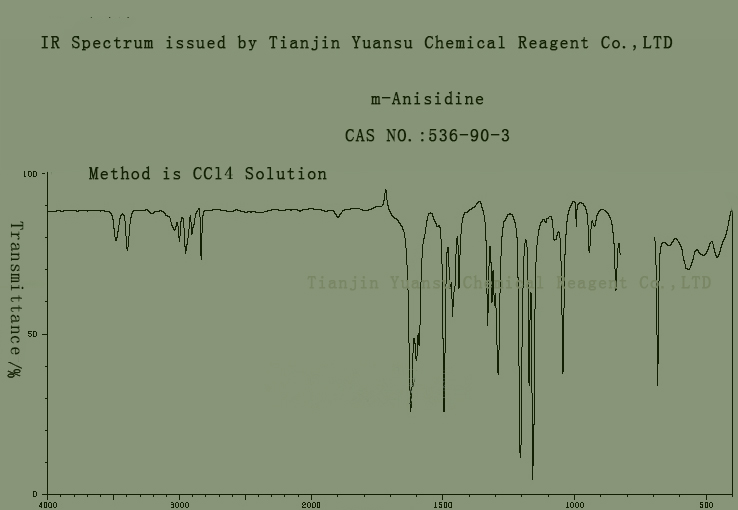
Assay: 99 min by GC
IR identity: conform
IR picture of M-Anisidine CAS NO.536-90-3 is as follows,
H-NMR Spectrum picture of M-Anisidine CAS NO.536-90-3 is as follows,
Reference of Article cited for your reference below,
(1)
Publication Name: Journal of Thermal Analysis and Calorimetry
Publication Date: 2024-07-08
DOI: 10.1007/s10973-024-13382-3
(2)
DOI: 10.1002/jhet.2406
Publication Date: 2016
Publication Name: J. Heterocyclic Chem.
(3)
DOI: 10.1002/jhet.2620
Publication Date: 2017
Publication Name: J. Heterocyclic Chem.